The role of piRNAs in planarian regeneration
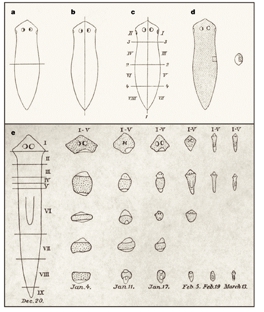
Classical description of planarian regeneration as described by Randolph and Morgan, 1897-98.
In one major focus of the lab, we investigate how piRNAs (PIWI-interacting RNAs), a complex class of small regulatory RNAs, contribute to the spectacular regeneration capacity of planarian flatworms. Intriguingly, two of the three planarian PIWI proteins are essential for proper stem cell function and regeneration of these animals. However, the exact mechanistic role piRNAs play during regeneration is unclear. We are thus investigating the role of planarian piRNAs during regeneration using molecular biology and systems biology approaches. Results from our lab unraveled an essential role of planarian piRNAs in global mRNA surveillance and the epigenetic control of transposable elements (Kim et al. 2019a, 2019b). Furthermore, we discovered a somatic piRNA pathway in the planarian epidermis and, more recently, an essential function of m6A in planarian biology (unpublished). Our long-term goal is to understand the connection between mechanisms regulating genome protection and transcription, including the piRNA and m6A pathways, and regeneration competence. This knowledge will also contribute to bringing us closer to be able to direct whole-organ regeneration in humans. Moreover, planarian piRNAs are enormously complex and thus are likely an impediment to stable transgene expression and transgenesis as such. Therefore, a deeper understanding of piRNA-mediated mRNA surveillance will contribute to paving the road towards stable planarian transgenesis.

Work on planarians in the Kuhn lab in 2023.
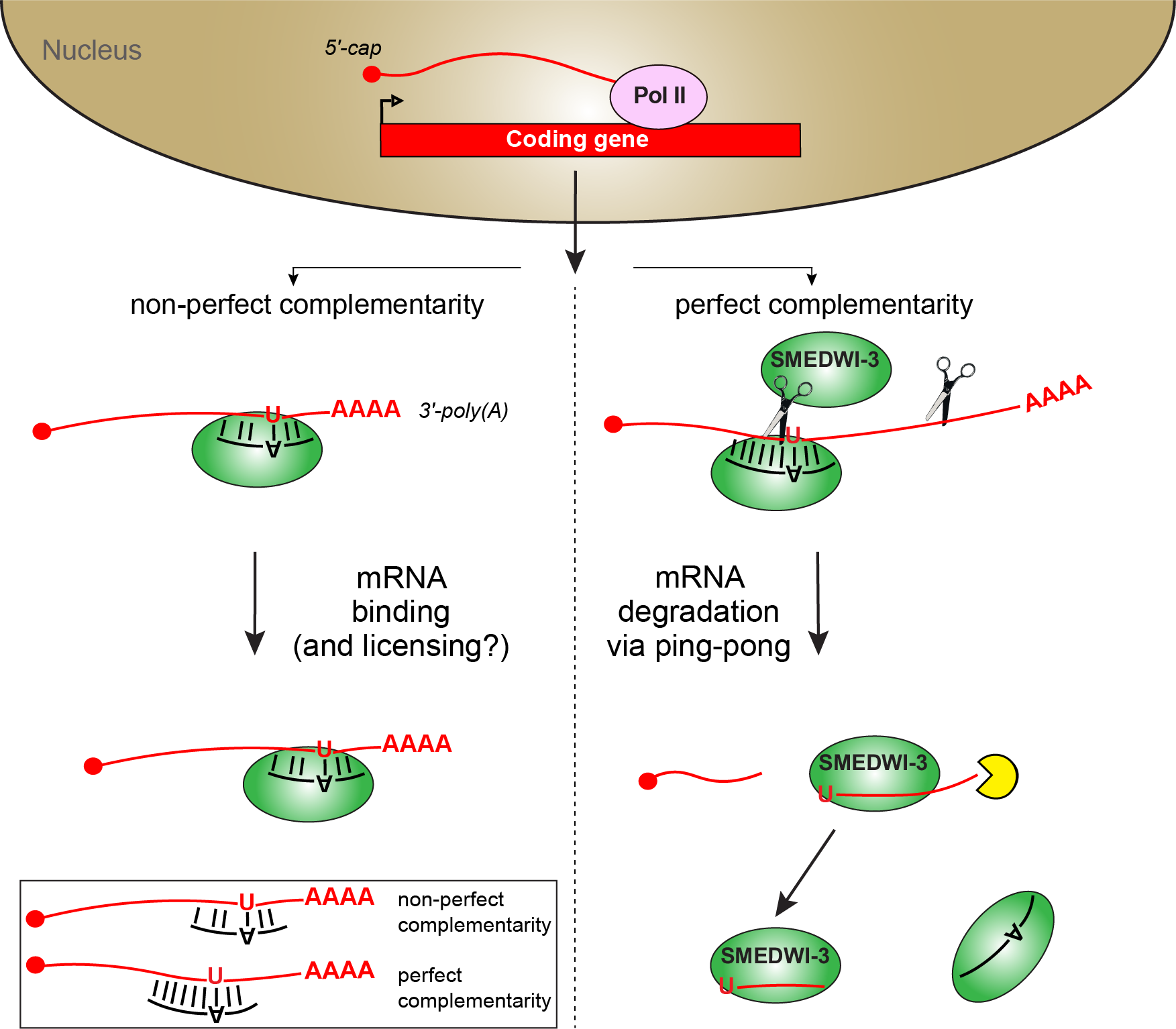
Working model of SMEDWI-3 as gatekeeper for mRNA fate in neoblasts, planarian stem cells (Kim et al., BiolChem 2020). While SMEDWI-3 degrades a set of mRNAs in a homotypical ping-pong cycle, it binds another set of mRNAs without processing them into piRNAs. Whether an mRNA is degraded or not is determined by the base-paring pattern between antisense piRNAs bound by SMEDWI-3 and target mRNAs.
Collaborators:
Elisabeth Duncan, University of Kentucky, Lexington, KY, USA


